אמנות הטיפול התרופתי בכאב בילדים המטו-אונקולוגיים - נייר עמדה - Pharmacological pain treatment in children hemato-oncology
ערך זה נמצא בבדיקה ועריכה על ידי מערכת ויקירפואה, וייתכן כי הוא לא ערוך ומוגה.
|
| |
|---|---|
| אמנות הטיפול התרופתי בכאב בילדים המטו-אונקולוגיים | |
| תחום | המטו-אונקולוגיה ילדים |

| |
| האיגוד המפרסם | איגודים משתתפים |
| קישור | באתר הר"י |
| יוצר הערך | העורכים |
| ניירות עמדה מתפרסמים ככלי עזר לרופא/ה ואינם באים במקום שיקול דעתו/ה בכל מצב נתון. כל הכתוב בלשון זכר מתייחס לשני המגדרים. | |
העורכים
- פרופ' אליעד דוידסון - יו"ר האגודה הישראלית לכאב, מנהל היחידה לשיכוך כאב, המרכז הרפואי הדסה עין-כרם
- ד"ר שיפרה אש - יו"ר האיגוד הישראלי להמטולוגיה ואונקולוגיה ילדים
- ד"ר איתי גור-אריה - מנהל המכון לשיכוך כאב, המרכז הרפואי ע"ש חיים שיבא, תה"ש
- ד"ר סילביו בריל - מנהל המכון לשיכוך כאב, המרכז הרפואי ת"א
- פרופ' פסח שוורצמן - יו"ר האיגוד הישראלי לרפואה פליאטיבית, מנהל יחידה פליאטיבית, המרכז הרפואי האוניברסיטאי סורוקה
- ד"ר גדי אבבה קמפינו - מנהל השרות לטיפול תומך ילדים, המערך להמטו-אונקולוגיה ילדים, ביה"ח לילדים ע"ש אדמונד ולילי ספרא, המרכז הרפואי ע"ש חיים שיבא, תה"ש
- ד"ר דניאל סטוקי - רופא מרדים ילדים, אחראי מרפאת כאב ילדים, מערך הרדמה, כאב וטיפול נמרץ, המרכז הרפואי ת""א
- ד"ר סרגי פוסטובסקי - סגן מנהלת מערך אונקולוגית ילדים, אחראי תחום גידולי מוח, סרקומות וטיפול תמיכתי, המרכז הרפואי רמב"ם
- ד"ר טל הראל - מומחה ברפואת ילדים ורופא כאב, המכון לרפואת שיכוך כאב, המרכז הרפואי ע"ש חיים שיבא, תה"ש
- ד"ר גיל גלעד - רופא בכיר, מחלקה המטו-אונקולוגית ילדים, מרכז שניידר לרפואת ילדים בישראל
איגודים משתתפים
- אגודה ישראלית לכאב
- האיגוד הישראלי לאונקולוגיה קלינית ורדיותרפיה
- האיגוד הישראלי להמטולוגיה ואונקולוגיה ילדים
- האיגוד לרפואה פליאטיבית בישראל
- האיגוד הישראלי לרפואה פנימית
- החוג לטיפול בכאב של איגוד רופאי המשפחה
- איגוד הכירורגים בישראל
- האיגוד הישראלי לאורתופדיה
- האיגוד הישראלי לפרמקולוגיה קלינית
CHRONIC & PERSISTENT CANCER PAIN / Dr Abebe-Campino Gadi
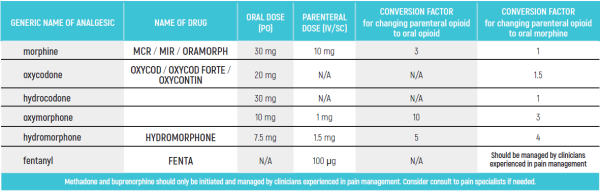
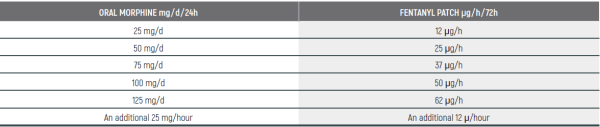
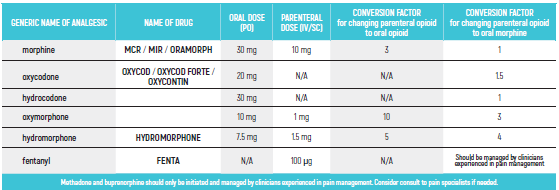
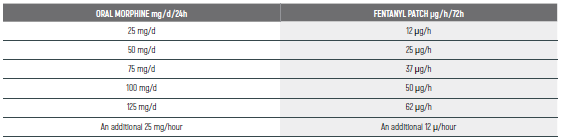
PEDIATRIC EQUIANALGESIC OPIOID DOSE CONVERSION - Table 18
The equianalgesic doses (oral and parenteral) can be affected by interpatient variability, type of pain (for example, acute versus chronic), chronic administration, and tolerance. The following table should serve only as a guide when switching from one opioid to another. It is recommended to reduce the dose of the new opioid by 30 to 50% to account for incomplete cross tolerance, and to periodically monitor for efficacy and adverse reactions and the dose adjusted accordingly.
- ביבליוגרפיה
- WHO guidelines on the pharmacological treatment of persisting pain in children with medical illnesses. WHO publication 2012. http://apps.who.int/iris/bitstream/handle/10665/44540/9789241548120_Guidelines.pdf;jsessionid=8BDAE35F2779DB0AEEAA683BA1F0FA02?sequence=1
- Erendira Vicencio-Rosas et al. Buprenorphine and pain treatment in pediatric patients: an update. J Pain Res. 2018; 11: 549-559
- de Leeuw T et al. The use of dipyrone [metamizol] as an analgesic in children: What is the evidence? A review. Paediatr Anaesth. 2017 Dec;27[12):1193-1201.
- Tutelman PR et al. Pain in Children With Cancer: Prevalence, Characteristics, and Parent Management. Clin J Pain. 2018 Mar;34[3):198-206
- Frederigue Rodieux et al. When the Safe Alternative Is Not That Safe: Tramadol Prescribing in Children. Front Pharmacol. 2018; 9:148.
- Drugs for chronic pain in children: A commentary on clinical practice and the absence of evidence. Gregoire MC, Finley GA. Pain Res Manag. 2013 Jan-Feb; 18(1): 47-50.
- Farber D, Hauer J & Duncan J. Pediatric Pain and Symptom Management Guidelines. Bridget Fowler Scullion, Pharm .2014. http://www.virtualhospice.ca/Assets/Boston%20Pediatric%20Pain%20&%20Symptom%20Management%20Guidelines%20-%202014_20170104132605.pdf
- Anderson MD. Cancer Pain - Pediatric. The practice algorithm (2017): 1-27. https://www.mdanderson.org/documents/for-physicians/algorithms/clinical-management/clin-management-cancer-pain-pedi-web-algorithm.pdf
- Anghelescu DL et al. Methadone use in children and young adults at a cancer center: A retrospective study. J Opioid Manag. 2011 ;7(5): 353-361.
- Bruera E & Sweeney C. Methadone use in cancer patients with pain: a review. J Palliat Med. 2002;5[1):127-38
- Capin0 AC, Miller JL & Johnson PN. Clonidine for Sedation and Analgesia and Withdrawal in Critically III Infants and Children. Pharmacotherapy 2016;36[12):1290-1299
- Anderson BJ et al. Tramadol: keep calm and carry on. Paediatr anaesth 2017; 27:785-78
- Friedrichsdorf SJ, Nugent AP & Strobl AQ. Codeine-associated pediatric deaths despite using recommended dosing guidelines: three case report. J Opioid Manag. 2013 Mar-Apr; 9[2):151-5
- Peterson DE, Bensadoun R-J, Roila F, ESMO Guidelines Working Group. Management of oral and gastrointestinal mucositis: ESMO Clinical Practice Guidelines. Ann. Oncol. 2011;22 Suppl 6:vi78-84.
- Laila RV, Sonis ST, Peterson DE. Management of oral mucositis in patients who have cancer. Dent. Clin. North Am. 2008;52:61-77- viii.
- Harris DJ. Cancer treatment-induced Mucositis Pain: strategies for assessment and management. Ther Clin Risk Manag 2006;2:251-258.
- James PJ, Howard RF & Williams DG. [2010] The addition of ketamine to a morphine nurse - or patient-controlled analgesia infusion [PCA/NCA] increases analgesic efficacy in children with Mucositis Pain. Pediatric Anesthesia, 20,805-811.
- Cohen SP, Bhatia A, Buvanendran A, et al. Consensus guidelines on the use of intravenous ketamine infusions for chronic pain from the American Society of Regional Anesthesia and Pain Medicine, The American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43[5): 521-546.
- Howard RF, Wiener S & Walker S M. Neuropathic pain in children. Archives of Disease in Childhood (2014); 99(1): 84-89.
- Krane E, Leong M, Golianu B, Leong Y. Treatment of Pediatric Pain with Nonconventional Analgesics. In: Schechter N, Berde C, Yaster M, editors. Pain in infants, children, and adolescents. Philadelphia: Lippincott Williams & Wilkins; 2003. pp. 225-240.
- McGrath P, Stevens B, Walker S, Zempsky W. Oxford textbook of paediatric pain. London: Oxford University Press, 2013.
MUCOSITIS PAIN / Dr Stocki Daniel
- ביבליוגרפיה
- http://www.mhra.gov.uk/Safetyinformation/DrugSafetyllpdate/CON088171
- de Leeuw TG, Dirckx M, Gonzalez Candel A, Scoones GP, Huygen FJPM, de Wildt SN. The use of dipyrone [metamizol] as an analgesic in children: What is the evidence? A review. Paediatr Anaesth 2017:27:1193-1201.
- Peterson DE, Bensadoun R-J, Roila F, ESMO Guidelines Working Group. Management of oral and gastrointestinal mucositis: ESMO Clinical Practice Guidelines. Ann. Oncol. 2011:22 Suppl 6:vi78-84.
- Pain Management in Mucositis at Sidney Children's Hospital - http://www.schn.health.nsw.gov.au/_policies/pdf/2014-7006.pdf
- Bensinger W, Schubert M, Ang K-K, Brizel D, Brown E, Eilers JG, Elting L, Mittal BB, Schattner MA, Spielberger R, Treister NS, Trotti AM. NCCN Task Force Report, prevention and management of mucositis in cancer care. J Natl Compr Cano Netw 2008:6 Suppl 1:51-21- guiz S22-4.
- Anderson BJ, Thomas J, Ottaway K, Chalkiadis GA. Tramadol: keep calm and carry on. Paediatr Anaesth 2017:27:785-788.
- BOZKURT P. Use of tramadol in children. Paediatr Anaesth 2005:15:1041-1047.
- Pendeville PE, Montigny Von S, Dort JR Veyckemans F. Double-blind randomized study of tramadol vs. paracetamol in analgesia after day-case tonsillectomy in children. Eur J Anaesthesiol 2 0 0 0:17:576- 582.
- Harris DJ. Cancer treatment-induced Mucositis Pain: strategies for assessment and management. Ther Clin Risk Manag 2006:2:251-258.
- Laila RV et al. Mucositis Guidelines Leadership Group of the Multinational Association of Supportive Care in Cancer and International Society of Oral Oncology [MASCO/ISOO]. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2014;120:1453-1461.
- Laila RV, Sonis ST, Peterson DE. Management of oral mucositis in patients who have cancer. Dent. Clin. North Am. 2008;52:61-77- viii.
- Zernikow B, Smale H, Michel E, Hasan C, Jorch N, Andler W. Paediatric cancer pain management using the WHO analgesic ladder-results of a prospective analysis from 2265 treatment days during a guality improvement study. 2006;10:587-595.
- Mason H, DeRubeis MB, Burke N, Shannon M, Karsies D, Wolf G, Eisbruch A, Worden E Symptom management during and after treatment with concurrent chemoradiotherapy for oropharyngeal cancer: A review of the literature and areas for future research. World J Clin Oncol 2016;7:220-226.
- James PJ, Howard RF & Williams DG. [2010] The addition of ketamine to a morphine nurse - or patient-controlled analgesia infusion [PCA/NCA] increases analgesic efficacy in children with Mucositis Pain. Pediatric Anesthesia, 20,805- 811.
- Firouzian A et al. Ultra-low-dose Naloxone as an Adjuvant to Patient Controlled Analgesia [PCA] With Morphine for Postoperative Pain Relief Following Lumber Discectomy: A Double-blind, Randomized, Placebo-controlled Trial. J Neurosurg Anesthesiol 2018 Jan; 30:26-31.
- Cepeda MS et al. Addition of ultralow dose naloxone to postoperative morphine PCA: unchanged analgesia and opioid reguirement but decreased incidence of opioid side effects. Pain. 2004 Jan;107[1-2):41-6.
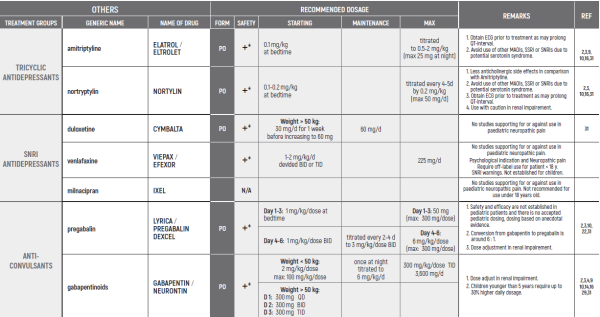
NEUROPATHIC PAIN / Dr Harel Tal
- ביבליוגרפיה
- WHO guidelines on the pharmacological treatment of persisting pain in children with medical illnesses, p.37, http:// apps.who.int/medicinedocs/documents/s19116en/s19116en.pdf. 2012. http://apps.who.int/iris/bitstream/handle/10665/44540/9789241548120_Guidelines.pdf;jsessionid=8BDAE35F2779DB0AEEAA683BA1F0FA02?sequence=1
- Friedrichsdorf, S. J. & Nugent, A. P. [2013). Management of neuropathic pain in children with cancer. Current Opinion in Supportive & Palliative Care 7(2): 131-138.
- Howard RF, Wiener S & Walker S M. Neuropathic pain in children. Archives of Disease in Childhood (2014); 99[1): 84-89.
- Haig GM, Bockbrader HN, Wesche DL, et al. Single-dose gabapentin pharmacokinetics and safety in healthy infants and children.) Clin Pharmacol. 2001;41[5):507-14.
- Finkel JC, Pestieau SR, Quezado ZM. Ketamine as an adjuvant for treatment of cancer pain in children and adolescents. J Pain. 2007;8[6):515-21.
- Blonk Ml, Koder BG, van den Bernt PM, Huygen FJ. Use of oral ketamine in chronic pain management: a review. Eur J Pain. 2010;14[5):466-72.
- Ugur F, Gulcu N, Boyaci A. Oral ketamine for pain relief in a child with abdominal malignancy. Pain Med. 2009;10[1):120-1.
- Anghelescu DL, Faughnan LG, Baker JN, Yang J, Kane JR. Use of epidural and peripheral nerve blocks at the end of life in children and young adults with cancer: the collaboration between a pain service and a palliative care service. Pediatric Anesthesia. [2011) 2010 Dec;20[12):1070-7.
- MD Anderson Pediatric Cancer Pain practice algorithm [2017) - https://www.mdanderson.org/documents/for- physicians/algorithms/clinical-management/clin-management-cancer-pain-pedi-web-algorithm.pdf
- Farber D, Hauer J & Duncan J. Pediatric Pain and Symptom Management Guidelines. Bridget Fowler Scullion, Pharm .2014. http://www.virtualhospice.ca/Assets/Boston%20Pediatric%20Pain%20&%20Symptom%20Management%20Guidelines%20 -%202014_20170104132605.pdf
- Bredlau, AL, Thakur R et al. Ketamine for pain in adults and children with cancer: a systematic review and synthesis of the literature. Pain Medicine 2013; 14[10):1505-1517.
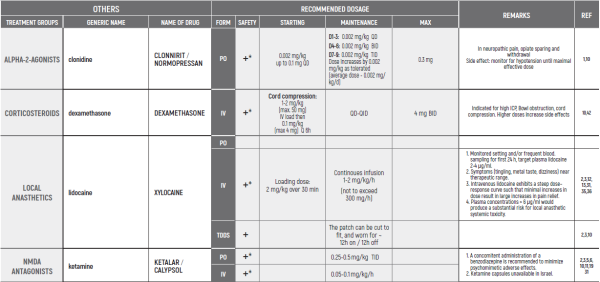
- Gibbons K, Niedner M et al. Continuous Lidocaine Infusions to Manage Opioid-Refractory Pain in a Series of Cancer Patients in a Pediatric Hospital. Pediatr Blood Cancer. 2016.
- Berde C, Koka A, Donado-Rincon C., Lidocaine Infusions and Other Options for Opioid-Resistant Pain Due to Pediatric Advanced Cancer. Pediatr Blood Cancer. 2016.
- Butkovic D, Toljan S, Mihovilovic-Novak B. Experience with gabapentin for neuropathic pain in adolescents: report of five cases. Pediatric Anesthesia 2006.
- Wallace MS, Lee J, Sorkin L. Intravenous lidocaine: effects on controlling pain after anti-GD2 antibody therapy in children with neuroblastoma-a report of a series. Anesth Analg 1997; 85:794-796.
- Krane E, Leong M, Golianu B, Leong Y. Treatment of Pediatric Pain with Nonconventional Analgesics. In: Schechter N, Berde C, Yaster M, editors. Pain in infants, children, and adolescents. Philadelphia: Lippincott Williams & Wilkins; 2003. pp. 225-240.
- Kachko L. Duloxetine contributing to a successful multimodal treatment program for peripheral femoral neuropathy and comorbid 'reactive depression' in an adolescent. Pain Res Manag. 2011.
- Burghardt KJ, Thomas ST, and Tutag-Lehr V. Off-label use of duloxetine for pediatric neuropathic pain. Off-Label Medications: Evidence-Based Use, Vol. 5, Iss. 6.
- Harrop EJ, Brombley K, Boyce K. Fifteen minute consultation: Practical pain management in paediatric palliative care. Arch Dis Child Educ Pract Ed. 2017.
- Rork JF, Berde CB, Goldstein RD. Regional anesthesia approaches to pain management in pediatric palliative care: a review of current knowledge. J Pain Symptom Manage. Dec 2013.
- Anghelescu D, Use of Epidural and Peripheral Nerve Blocks at End of Life in Children and Young Adults with Cancer: The Collaboration between a Pain Service and a Palliative Care Service. Paediatr Anaesth 2010.
- Vondracek R Oslejskova H, Kepak T, et al. Efficacy of pregabalin in neuropathic pain in paediatric oncological patients. Eur J Paediatr Neurol. 2009;13:332-6.
- Golden AS, Haut SR, Moshe SL. Nonepileptic uses of antiepileptic drugs in children and adolescents. Pediatr Neurol. 2006;34:421-32.
- Nicholson AB. Methadone for cancer pain. Cochrane Database of Systematic Reviews 2007.
- Anghelescu DL, et al. Methadone use in children and young adults at a cancer center: a retrospective study. J Opioid Manag. 2011.
- Madden K, et al. Very-Low-Dose Methadone To Treat Refractory Neuropathic Pain in Children with Cancer. J Palliat Med. 2017.
- Madden K, Mills S, Dibaj S, Williams J L, Liu D, Bruera E. Methadone as the Initial Long-Acting Opioid in Children with Advanced Cancer. J Palliat Med 2018.
- Cooper et al. Non-steroidal anti-inflammatory drugs [NSAIDs] for cancer-related pain in children and adolescents. Cochrane Database of Systematic Reviews 2017 Jul 24;7:CD012563, Issue 7.
- J Collins. Cancer pain management in children. European journal of Pain (2001).
- Constance et al. Pharmacokinetics, pharmacodynamics and pharmacogenetics associated with nonsteroidal anti- inflammatory drugs and opioids in pediatric cancer patients.
- McGrath R Stevens B, Walker S, Zempsky W. Oxford textbook of paediatric pain. London: Oxford University Press, 2013.
- Derry et al. Fentanyl for neuropathic pain in adults - Cochrance review. [2016] https://www.cochrane.org/CD011605/ SYMPT_fentanyl-neuropathic-pain-adults.
- Vicencio-Rosas et al, Buprenorphine and pain treatment in pediatric patients: an update [J Pain Res 2018).
- Duehmke et al, Tramadol for neuropathic pain - Cochrane review (2010).
- IV Lidocaine for Neuropathic Pain - Lucile Packard Children's Hospital Stanford (2014).
- Guidelines for Low Dose Continuous Infusion Lidocaine for Pain in Pediatric Patients - St. Louis Childrens Hospital.
- de Leeuw T et al. The use of dipyrone [metamizol] as an analgesic in children: What is the evidence? A review. Paediatr Anaesth. 2017 Dec;27[12):1193-1201.
- Erendira Vicencio-Rosas et al. Buprenorphine and pain treatment in pediatric patients: an update. J Pain Res. 2018; 11: 549-559.
- Frederique Rodieux et al. When the Safe Alternative Is Not That Safe: Tramadol Prescribing in Children. Front Pharmacol. 2018; 9:148.
- Peterson DE, Bensadoun R-J, Roila F, ESMO Guidelines Working Group. Management of oral and gastrointestinal mucositis: ESMO Clinical Practice Guidelines. Ann. Oncol. 2011;22 Suppl 6:vi78-84.
- Laila RV, Sonis ST, Peterson DE. Management of oral mucositis in patients who have cancer. Dent. Clin. North Am. 2008;52:61-77- viii.
- Capino AC, Miller JL & Johnson PN. Clonidine for Sedation and Analgesia and Withdrawal in Critically III Infants and Children. Pharmacotherapy 2016;36[12):1290-1299.
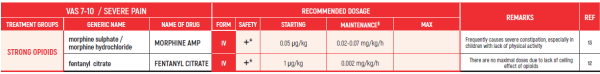
END OF LIFE / Dr Postovsky Seregey
This chapter refers only to pain conditions in pediatric oncology patients which accompanied by unbearable suffering at the end of life and when all usually used drugs in conventional doses have been proven non-effective.
This type of treatment of pain management is referred to as palliative sedation. All drugs are given intravenous.
This chapter refers only to pain conditions in pediatric oncology patients which accompanied by unbearable suffering at the end of life and when all usually used drugs in conventional doses have been proven non-effective.
This type of treatment of pain management is referred to as palliative sedation. All drugs are given intravenous.
- ביבליוגרפיה
- Korzeniewska-Eksterowicz A, Przysto t, Fendler W, Stolarska M, Mfynarski W. Palliative sedation at home for terminally ill children with cancer. J Pain Symptom Manage 2014; 48: 968-974.
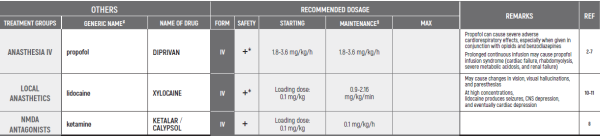
- Anghelescu DL, Hamilton H, Faughnan LG, Johnson LM, Baker JN. Pediatric palliative sedation therapy with propofol: recommendations based on experience in children with terminal cancer. J Palliat Med. 2012; 15: 1082-90.
- Hooke MC, Grund E, Quammen H, Miller B, McCormick P Bostrom B. Propofol use in pediatric patients with severe cancer pain at the end of life. J Pediatr Oncol Nurs 2007; 24: 29-34.
- Fodale V, La Monaca E: Propofol infusion syndrome: An overview of a perplexing disease. Drug Safety 2008; 31: 293-303.
- Vasile B, Rasulo F, Candiani A, Latronico N. The pathophysiology of propofol infusion syndrome: a simple name for a complex syndrome. Intensive Care Med 2003; 29: 1417-25.
- Bray RJ. Propofol infusion syndrome in children. Paediatric anaesthesia. 1998; 8: 491-9.
- Parke TJ, Stevens JE, Rice AS, Greenaway CL, Bray RJ, Smith PJ, et al. Metabolic acidosis and fatal myocardial failure after propofol infusion in children: five case reports. Bmj. 1992; 305: 613-6.
- Tsui BC, Davies D, Desai S, Malherbe S. Intravenous Ketamine Infusion as an Adjuvant to Morphine in a 2-Year-Old with Severe Cancer Pain from Metastatic Neuroblastoma. J Pediatr Hematol Oncol. 2004; 26: 678-80.
- Postovsky S, Moaed B, Krivoy E, Ofir R, Ben Arush MW. Practice of palliative sedation in children with brain tumors and sarcomas at the end of life. Pediatr Hematol Oncol. 2007; 24: 409-15.
- Gibbons K, DeMonbrun A, Beckman EJ, Keefer P, Wagner D, Stewart M, et al. Continuous Lidocaine Infusions to Manage Opioid-Refractory Pain in a Series of Cancer Patients in a Pediatric Hospital. Pediatr Blood Cancer. 2016; 63: 1168-74.
- Berde C, Koka A, Donado-Rincon C. Lidocaine Infusions and Other Options for Opioid-Resistant Pain Due to Pediatric Advanced Cancer. Pediatr Blood Cancer. 2016; 63: 1141-3.
- Hauer J, Duncan J, Fowler Scullion B. Pediatric Pain and Symptom Management Guidelines, Boston, MA. 2014.
- Rodrigues A, Wong C, Mattiussi A, Alexander S, Lau E, Dupuis LL. Methylnaltrexone for opioid-induced constipation in pediatric oncology patients. Pediatr Blood Cancer. 2013; 60: 1667-70.
- Amrein R, Hetzel W. Pharmacology of Dormicum (midazolam) and Anexate (flumazenil). Acta Anaesthesiol Scand Suppl. 1990; 92: 6-15.
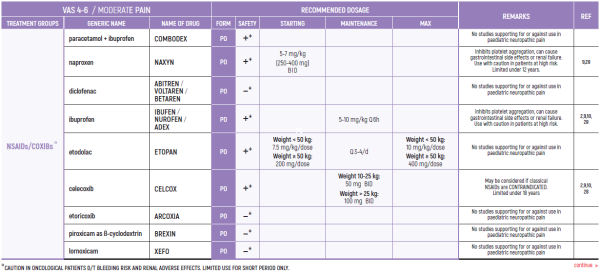
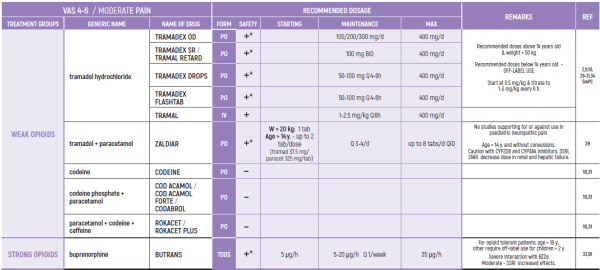
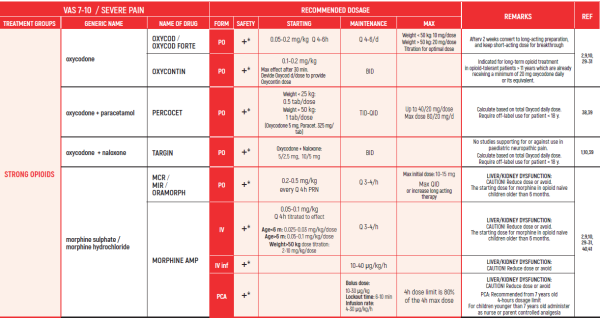
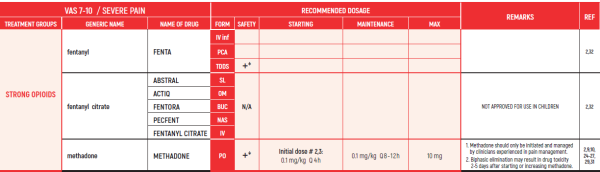
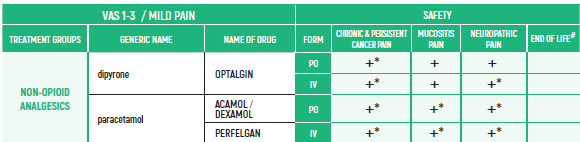
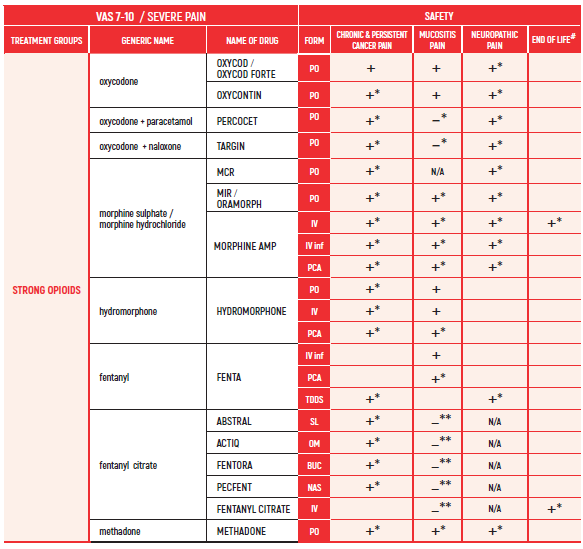
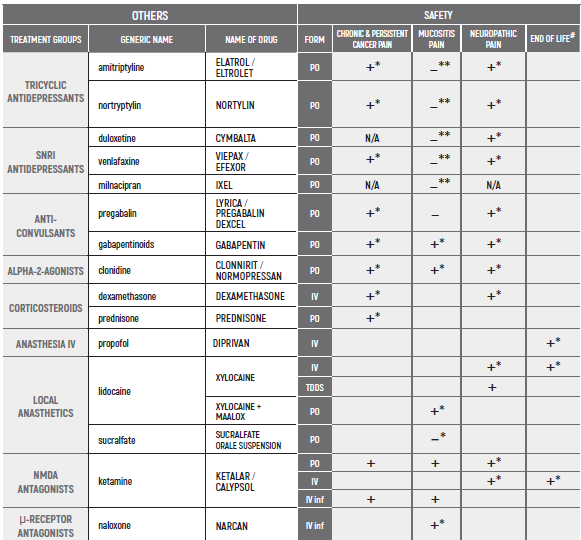
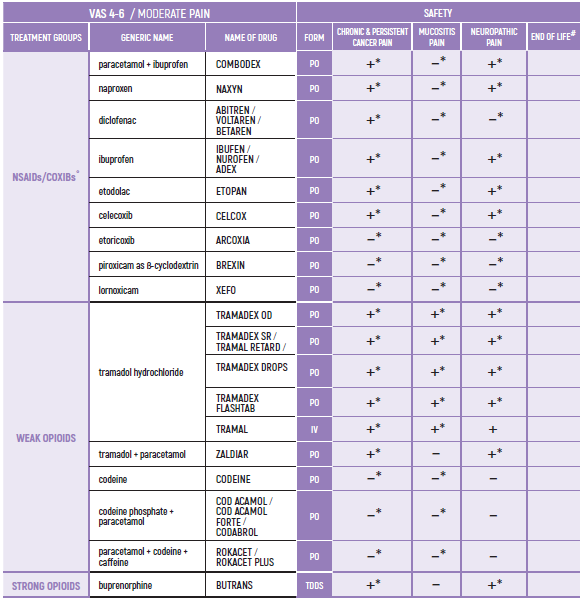
PHARMACOLOGICAL PAIN TREATMENT INCHILDRENHEMATO-ONCOLOGY
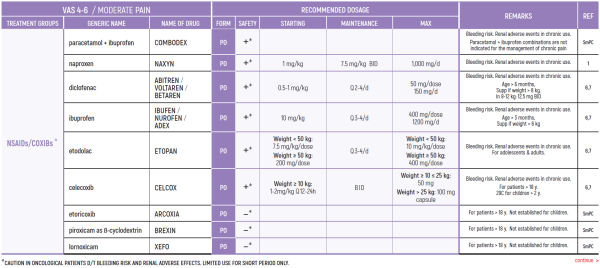
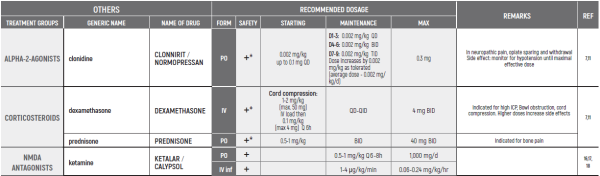
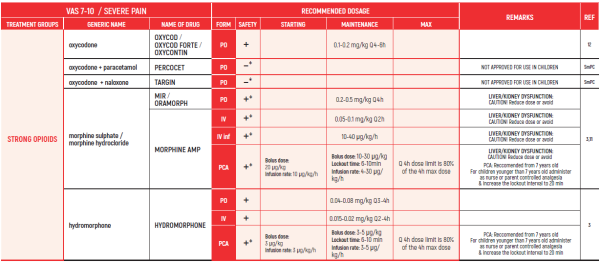
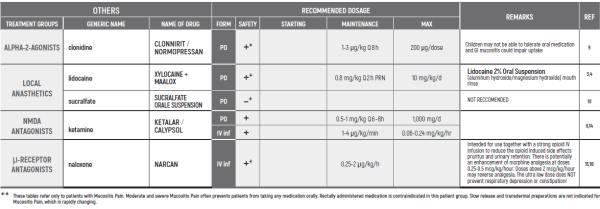
°CAUTION IN ONCOLOGICAL PATIENTS D/T BLEEDING RISK AND RENAL ADVERSE EFFECTS. LIMITED USE FOR SHORT PERIOD ONLY.
(#) This chapter refers only to pain conditions in pediatric oncology patients which accompanied by unbearable suffering at the end of life and when all usually used drugs in conventional doses have been proven non-effective. This type of treatment of pain management is referred to as palliative sedation. All drugs are given intravenous.
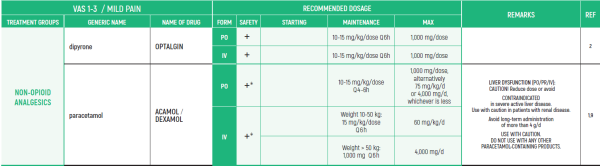
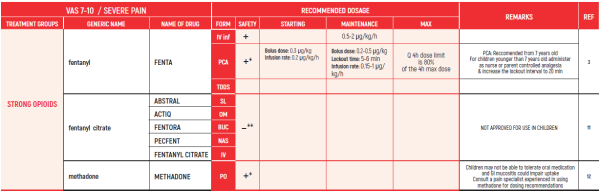
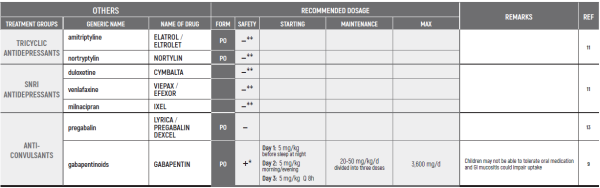
(* *) These tables refer only to patients with Mucositis Pain. Moderate and severe Mucositis Pain often prevents patients from taking any medication orally. Rectally administered medication is contraindicated in this patient group. Slow release and transdermal preparations are not indicated for Mucositis Pain, which is rapidly changing.
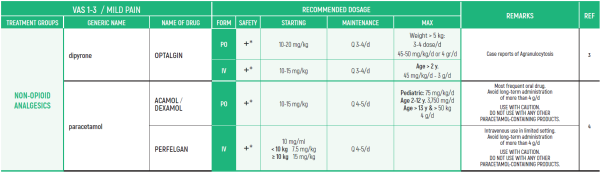
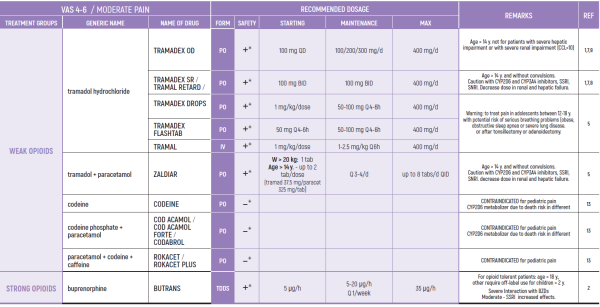
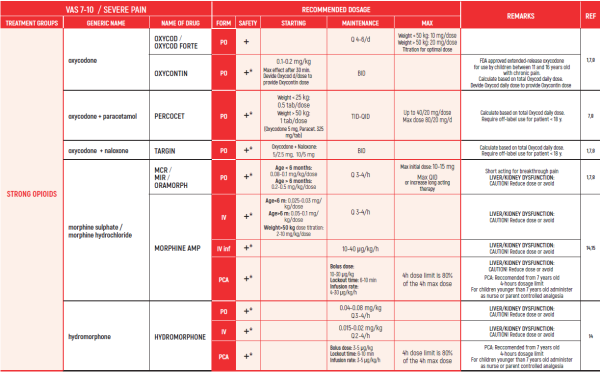
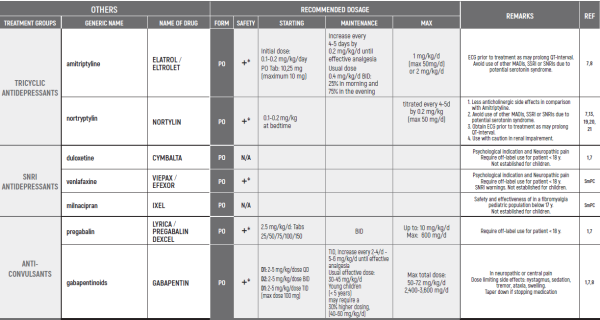
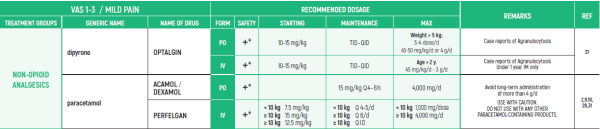
ALL PRODUCT USE ARE BY SmPC BELLOW THE RECOMMENDED TREATMENT AGE - OFF-LABEL USE.
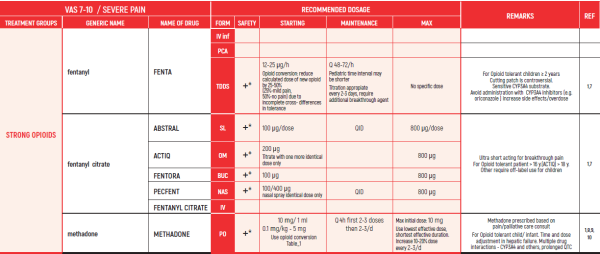
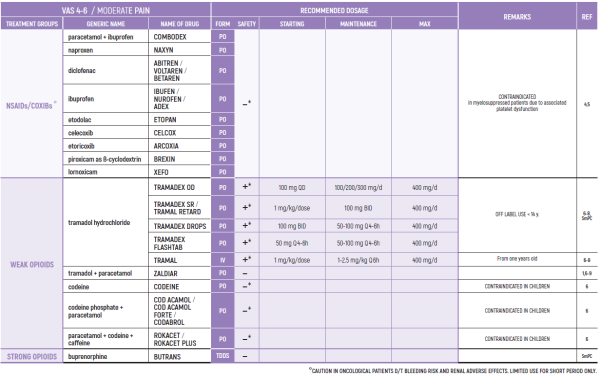
THE WHO CONCEPT OF “THREE-STEP LADDER OF PAIN RELIEF” (1986) CAN BE MODIFIED IN CHILDREN TO THE TWO STEP STRATEGY (WHO 2012): PAIN CONTROL IS PLANNED “AROUND THE CLOCK” WITH OPIOID TITRATION BASED ON PAIN SEVERITY AND PERSONAL RESPONSE IN MODERATE PAIN CONSIDER THE USE OF LOW DOSE STRONG OPIATES TO RELIEF PAIN. LOW DOSE OPIATES ARE EFFECTIVE THERAPY FOR MODERATE PAIN, CONSIDERING THE LIMITED AVAILABLE WEAK OPIATES.
(WHO guidelines on the pharmacological treatment of persisting pain in children with medical illnesses. WHO publication 2012)
Pediatric Equianalgesic Opioid Dose Conversion - Table 1 Dr Abebe-Campino G.
The equianalgesic doses (oral and parenteral) can be affected by interpatient variability, type of pain (for example, acute versus chronic), chronic administration, and tolerance. The following table should serve only as a guide when switching from one opioid to another. It is recommended to reduce the dose of the new opioid by 30 to 50% to account for incomplete cross tolerance, and to periodically monitor for efficacy and adverse reactions and the dose adjusted accordingly.


 כניסה
כניסה  עקבו אחרינו בפייסבוק
עקבו אחרינו בפייסבוק